How to Draw a Black Wallnut Tree Leaf
Juglans nigra L.
Black Walnut
Juglandaceae -- Walnut family
Robert D. Williams
Black walnut (Juglans nigra), also called eastern black walnut and American walnut, is one of the scarcest and most coveted native hardwoods. Small natural groves frequently found in mixed forests on moist alluvial soils have been heavily logged. The fine straight-grained wood made prize pieces of solid furniture and gunstocks. As the supply diminishes, the remaining quality black walnut is used primarily for veneer. The distinctive tasting nuts are in demand for baked goods and ice cream, but people must be quick to harvest them before the squirrels. The shells are ground for use in many products.
Habitat
Native Range
Black walnut typically grows as scattered individual trees or in small groups throughout the central and eastern parts of the United States. Although it is found on a variety of sites, black walnut grows best on good sites in coves and well-drained bottoms in the Appalachians and the Midwest. Its natural range extends from western Vermont and Massachusetts west through New York to southern Ontario, central Michigan, southern Minnesota, eastern South Dakota and northeastern Nebraska; south to western Oklahoma and central Texas; excluding the Mississippi River Valley and Delta, it ranges east to northwestern Florida and Georgia (28,29). On the western fringe of its range in Kansas, walnut is fairly abundant and frequently makes up 50 percent or more of the basal area in stands of several hectares (21).
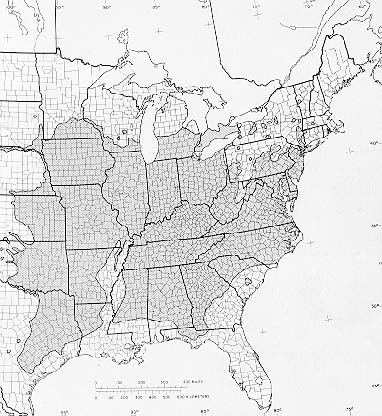
-The native range of black walnut.
Climate
The growing season within the range of black walnut ranges from 140 days in the north to 280 days in western Florida (10,43). Annual precipitation is less than 640 mm (25 in) in northern Nebraska and 1780 mm. (70 in) or more in the Appalachians of Tennessee and North Carolina. Mean annual temperatures range from about 7° C (45° F) in the north to 19° C (67° F) in the south. Temperatures as low as -43° C (-45° F) have occurred where walnut grows, but few races of black walnut can tolerate such low temperature. Within black walnut's optimum range, the average annual temperature is about 13° C (55° F), the frost-free season is at least 170 days, and the average annual precipitation is at least 890 min (35 in).
Soils and Topography
Black walnut is sensitive to soil conditions and develops best on deep, well-drained, nearly neutral soils that are generally moist and fertile (10). These soils are in the orders Alfisols and Entisols. Although an Ohio study indicated that site index for black walnut was not significantly related to pH values between 4.6 and 8.2, site index was highest on limestone derived soils even though some of the soils were acid. Walnut grows best on sandy loam, loam, or silt loam textured soils but also grows well on silty clay loam soils (31). Soils with these textures hold a large amount of water that is available to the tree during dry periods of the growing season. Internal drainage and depth to gravel are especially important site characteristics for black walnut. On well-drained soils, 76 cm (30 in) or more to mottling, 25-year-old trees were 6.6 cm (2.6 in) larger in d.b.h. than trees growing on imperfectly drained soils, 15 to 76 cm (6 to 30 in) to mottling. Twenty-five-year-old trees on deep soils, more than 102 cm (40 in) from surface to gravel, were 5.2 m (17 ft) taller and 6.4 cm (2.5 in) larger in d.b.h. than trees on shallow soils less than 102 cm (40 in) from surface to gravel (30).
Walnut is common on limestone soils and grows especially well on deep loams, loess soils, and fertile alluvial deposits. It also grows well on good agricultural soils that do not have fragipans. Walnut grows slowly on wet bottom land and on sandy or dry ridges and slopes. Throughout its range, walnut generally reaches its greatest size and value along streams and on the lower portion of north- or east-facing slopes. This is particularly true near the limits of its natural range. In northeastern Kansas, site index on alluvial soils was 2.4 rn (8 ft) greater than on residual soils and 2.7 in (9 ft) greater on northeast than on southwest aspects (20).
Associated Forest Cover
Black walnut grows in many of the mixed mesophytic forests but is seldom abundant (43). Usually it is found scattered among other trees; pure stands are rare, small, and usually located on the forest edge. Black walnut is a common associate in five forest cover types (16): Sugar Maple (Society of
American Foresters Type 27) in the central hardwood zone and the Appalachian highlands, Yellow-Poplar (Type 57) at lower elevations of the Appalachians, Yellow-Poplar-White Oak-Northern Red Oak (Type 59) at lower elevations, Beech-Sugar Maple (Type 60) in the Midwest, and Silver Maple-American Elm (Type 62) in southern Ontario washboard swamps where high and low ground intermingle.
It is also found as an occasional associated species in four cover types: Chestnut Oak (Type 44), White Oak-Black Oak-Northern Red Oak (Type 52), Northern Red Oak (Type 55) on moist sites, and Sassafras-Persimmon (Type 64) in older stands.
Chief associated species include yellow-poplar (Liriodendron tulipifera), white ash (Fraxinus americana), black cherry (Prunus serotina), basswood (Tilia americana), beech (Fagus grandifolia), sugar Maple (Acer saccharum), oaks Quercus spp.), and hickories (Carya spp.). Near the western edge of its range, black walnut may be confined to floodplains, where it grows either with American elm (Ulmus americana), hackberry (Celtis occidentalis), green ash (Fraxinus pennsylvanica), and boxelder (Acer negundo), or with basswood and red oak Quercus rubra) on lower slopes and other favorable sites (10).
No universal vegetative indicator of a good walnut site is known, but the presence of Kentucky coffeetree (Gymnocladus dioicus) seems to indicate such a site (10,43). In general, where yellow-poplar, white ash, red oak, basswood, sugar maple, or slippery elm (Ulmus rubra) grow well, black walnut thrives also.
An antagonism between black walnut and many other plants growing within its root zone has been recognized and is attributed to juglone, a toxic substance found in the leaves, bark, nut husks, and roots of walnut trees (32,42). Some tree species apparently are immune, but others, such as paper birch (Betula Papyrifera), red pine (Pinus resinosa), white pine (P. strobus), Scotch pine (P. sylvestris), and apple (Malus spp.), reportedly are sensitive. Tomatoes are especially susceptible. In a laboratory study, juglone at high concentrations was lethal to four coniferous species, but seedling growth was actually promoted when exposed to minute concentrations (19). Although tomatoes are especially susceptible to juglone, black walnut trees may be compatible with some agricultural crops and might even improve the growth of bluegrass (Poa spp.).
Life History
Reproduction and Early Growth
Flowering and Fruiting- Depending on latitude, black walnut flowers generally begin to appear about mid-April in the South and progressively later until early June in the northern part of the natural range. Flowering and leafing out occur at approximately the same time and always early enough for possible damage by late spring frosts (18,27).
Walnut is monoecious; male flowers, which are slender catkins, develop from axillary buds on the previous year's outer nodes, while female flowers occur in short terminal spikes, ranging from a few to many, home on the current year's shoots. Flowering is dichogamous, and protogyny (the female flowers appearing first) is more common than protandry (male flowers appearing first) (33,34). Because of its dichogamous flowering habit, self-pollination is unlikely. However, individual trees usually are not self-sterile; if they are not pollinated by neighboring trees, they may set self-fertilized seeds (3). Fertilization follows 2 to 5 days after pollination, succeeded by development of the husk, the shell, and finally by the seed itself (18).
Seed Production and Dissemination- The large edible nut ripens in September or October of the same year and drops shortly after the leaves fall. Good seed crops are produced irregularly, perhaps twice in 5 years. Open-grown trees may produce some seed when only 4 to 6 years old, but large seed crops do not occur until the trees are 20 to 30 years old (28). For example, at 10 years of age, a midwest plantation produced 28 kg of hulled nuts per hectare (25 lbs/acre), and by age 12 production had increased to 112 kg/ha (100 lb/acre). Best seed production begins when the trees are about 30 years old and continues for another 100 years. Seed is disseminated only short distances by gravity and animals.
In a Missouri study, seed production of trees about 28 years old and 19.3 cm (7.6 in) in d.b.h. was nearly doubled by release and fertilization (40). Trees released but not fertilized produced 13 percent more nuts than nonreleased trees.
Stratification for 90 to 120 days is required for optimum seed germination but the necessity and duration of stratification may vary by seed source (46). In Canada, 69 to 81 percent of nuts stratified 19 months germinated within 3 weeks of seeding, while 10 to 25 percent of nuts stratified 7 months germinated after 12 weeks (48). When nuts that had not germinated after 12 weeks in the seedbed following 7 months stratification were stratified for an additional 9 months, 81 percent germinated within 3 weeks. Many of the nuts stratified for 31 months germinated while in storage.
Seedling Development- Germination is hypogeal (46). Young black walnut seedlings are intolerant of shade and are seldom found under dense tree canopies. Regeneration develops primarily from seed that squirrels bury and fail to recover. Normal winter temperatures usually cause the buried seeds to break dormancy the following spring, but germination is sometimes delayed until the second year.
Seedlings emerge in April or May the first or second spring after the seed is planted (46). On deep, rich, moist soils in coves or well-drained bottom land, seedlings may grow 91 cm (36 in) the first year and even more the second growing season. Although black walnut does not make as rapid height growth as yellow-poplar and white ash on good sites, it generally surpasses the oaks. In eastern Nebraska, near the western edge of its range, walnut made much better height growth than oaks or basswood on a prairie site (10). Walnut developed an excellent root system and was several times taller than the other tree species.
Height growth begins slowly in the spring, reaches a peak rate in late April and May, and is complete by the middle of July or the first of August. Black walnut loses its leaves somewhat earlier than other trees and has a growing period of from 115 to 135 days (10).
Because of its large taproot, planted walnut seedlings typically survive well. However, they require weed control during the first 2 or 3 years to grow well (26).
Vegetative Reproduction- If small black walnut trees are cut or killed back by fire, the stumps usually sprout. Sprouts originating near the root collar generally are free from defect but sprouts originating high on older stumps often develop heart rot or other decay from the parent stump.
Within the last few years the success of grafting and budding of walnut has increased substantially. From 80 to 100 percent success has been achieved by three grafting methods done in the greenhouse and growth chamber (35). In the field the success rate for inlay grafting, the best method tested, ranged from 33 to 83 percent. A consistent field survival of 70 to 90 percent for the outplantings of grafted stock is predicted if tested procedures are followed (4). Black walnut is compatible with several other Juglans species, either as a root stock or scion (22).
Sapling and Pole Stages to Maturity
Growth and Yield- On the best sites, young black walnut trees may grow 91 to 122 cm (36 to 48 in) in height per year (28). The best tree in a southern Indiana plantation at age 7 was 11.9 cm (4.7 in) d.b.h. and 7.6 in (25 ft) in height (9). In a southern Illinois plantation (site index 24.4 m or 80 ft at base age 50 years), the best tree was 21 cm (8.3 in) d.b.h. and 12 m (40 ft) tall at age 14 (1). However, the average size tree in the plantation was 12 cm (4.8 in) d.b.h. and 7 m (24 ft) tall. Even on less favorable sites (site index 21.3 m or 70 ft), trees reach heights of 12 to 15 rn (40 to 50 ft) and diameters of 15 to 25 cm (6 to 10 in) in 20 years (28). In contrast, diameter growth of black walnut planted on Kansas strip mine spoil banks averaged only 6 mm (0.25 in) per year and height growth averaged only 33.5 cm (13.2 in) per year during the first 10 to 12 years (10). On Illinois spoil banks trees grew best on the lower slopes, on areas formed from limestone parent material and containing a high percentage of fine soil, or if underplanted with black locust (Robinia pseudoacacia). In two 10-year-old southern Illinois plantations, walnut trees in mixture with autumn-olive (Elaeagnus umbellata), a nitrogen-fixing species, were 89 percent taller and 104 percent larger in diameter than walnut trees in pure walnut plots (41). In an Indiana study, 10 years after autumn-olive was interplanted into 2-year-old black walnut, the walnut in the interplanted plots were 2.6 rn (8.4 ft) taller than those in the pure plots (14).
Mature black walnut trees on good sites may reach 30 to 37 m (100 to 120 ft) in height and 76 to 102 cm (30 to 40 in) in d.b.h. (28). Trees 40 m (130 ft) tall and more than 244 cm (96 in) in d.b.h. have been reported in Wisconsin. In Indiana, black walnut trees were 46 m (150 ft) tall and 183 cm (72 in) in d.b.h. on the most favorable sites (43). Research and experience indicate that with proper care it may be possible to produce 41-cm (16-in) saw logs in 30 to 35 years, and by planting on good sites it may be possible to produce 51 cm (20 in) veneer logs in 40 to 50 years. By applying some basic cultural practices, such as release and pruning, to established trees, growth and quality can be greatly increased in only a few years.
Board-foot volume growth rate was correlated with site quality in midwestern plantations. According to Kellogg's yield tables (23), predicted yield for site index 21.3 rn (70 ft) at age 75 is 10 times that of site index 12.2 m (40 ft), and yields for site index 18.3 m (60 ft) are twice those for site index 15.2 m (50 ft). The yield tables also show that periodic annual growth rate is not constant: maximum growth occurs between ages 40 and 50 years on the better sites.
Rooting Habit- The root system of mature black walnut has been described as combining the deep taproot of more xeric trees, such as the oaks, with the strong laterals characteristic of more mesic ones, such as maple. The rooting configuration of individual trees depends on soil texture and moisture conditions (47).
The root system is deep and wide spreading, with a definite taproot, at least in early life. The taproot of a 9-year-old walnut tree excavated from an Indiana plantation was 2.3 rn (7.5 ft) long and the lateral roots extended more than 2.4 m (8 ft) from the taproot (11). One-year-old walnut seedlings lifted from nursery seedbeds have well-developed taproots (51). The mass of fibrous roots varies with the soil type; the more fibrous-rooted seedlings develop in the more sandy-textured soils.
Early growth of the seedling root system is rapid. Vertical taproot extension during the first growing season is great, especially on drier soils. One researcher reported a taproot penetration of more than 1.2 rn (4 ft) for 1-year-old walnut seedlings on a prairie silt loam soil. Another reported 64 to 69 cm (25 to 27 in) for 1-year-old walnut on a more moist site (47). In the second year of root growth, the taproot continues to extend and many lateral roots develop.
The depth of walnut lateral roots may vary in response to root competition with its associates. In one study, lateral roots of walnut occupied a much shallower position in pure walnut stands than in mixed walnut-ash stands. This was explained by theorizing that the ash, having a strongly developed surface root system, forced the walnut roots into deeper soil layers. Root competition with Norway maple (Acer platanoides), on the other hand, was not as intense (47).
Black walnut is moderately tolerant of flooding. Mature trees are generally killed after 90 days of continuous inundation during the growing season, although some individuals may survive for 150 days or more. Black walnut is more flood-tolerant than black cherry, shortleaf pine (Pinus echinata), basswood, and shagbark hickory (Carya ovata) (47).
The initial root form of black walnut, with its rapidly growing juvenile taproot and wide spreading laterals, is characteristic of species that grow on deep, fine-textured soils in regions with well-distributed summer rains. Such soils maintain a fairly uniform available water content to considerable depth, and walnut growing on these soils are able to draw their moisture and nutrients largely from the more fertile shallow soil while still being able to rely on the deeper soil layers for survival during times of drought.
Black walnut forms endomycorrhizae of the vesicular-arbuscular type. One study revealed that 100 percent of the walnut seedlings grown in a southern Michigan nursery had endomycorrhizae, but seedlings grown in a southern Indiana nursery had no mycorrhizae. A recent study shows that several Glomus species form a symbiotic relation with black walnut seedlings. Some Glomus, species and combinations of species increased growth of black walnut (36).
Reaction to Competition- Black walnut is classed as intolerant of shade (2). In mixed forest stands it must be dominant or codominant to survive, although it has survived and grown in the light shade of black locust. In a mixed hardwood stand in Indiana, pole-size black walnut responded to crown release by more than doubling diameter growth over a 10-year period (39,40). Trees only partially released grew about 50 percent more than unreleased trees. Controlling understory growth had little effect on growth of the walnut trees. Following release, dominant and codominant trees continue to grow more rapidly than those in intermediate or suppressed crown classes, but strong intermediates often respond most to release (in terms of growth rate increase). A walnut tree should be considered for release if it is healthy, has a bole with potential to make a veneer or high quality saw log, and is small enough that it can reasonably be left for at least 10 more years. To be effective, release must be thorough. A rule of thumb is that at least threefourths of the crown of the released tree should be at least 1.5 m (5 ft) from the crowns of adjacent trees 60 to 100 percent as tall, and at least 3 m (10 ft) from the crowns of taller trees. Subsequent releases will be required at intervals of 6 to 10 years to maintain free growing space.
Some bole sprouting can be expected on forest-grown trees that are released for the first time. Bole sprouts developed on almost half of the unreleased trees and on almost two-thirds of the released trees during an Indiana study (39). Sprouts were more numerous on the unreleased trees (16.1 sprouts per tree) than on the partially (12.2 sprouts per tree) and completely released trees (9.2 sprouts per tree), but the sprouts were much larger on the released trees. The intermediate and suppressed trees had more sprouts than dominant or codominate trees. Most of the bole sprouts were above the butt log, and more were on the south side than on the north side of the trees.
Control of competing vegetation is especially important in new plantations. In an Indiana study, walnut seedlings established on formerly cultivated fields and given 3 years of weed control were 100 cm (39 in) taller at 10 years of age, and 15 mm (0.6 in) larger d.b.h. than trees given 2 years of control (53). Trees with vegetation controlled 2 years were 40 cm (15.7 in) taller and 5 mm (0.2 in) larger in diameter than those where weeds were controlled only 1 year. Broadcast weed control is neither necessary nor desirable because it aggravates erosion problems.
In a southern Illinois experiment, seventh-year survival of black walnut planted on a cleared forest site was 94 to 99 percent regardless of weed control treatment (25). The young trees grew better, however, when all vegetation or only forbs and grasses were controlled than when only woody vegetation was controlled or when no vegetation control was used. Biennial control was no better than triennial, but annual control was superior. When only woody vegetation was controlled, frequency of treatment had no effect.
Pruning lateral branches helps to produce knot-free wood under open growing conditions that would normally permit most of the lower branches to persist. The objective of pruning is to produce a clear bole while minimizing damage to the tree and growth loss. When needed, pruning should be begun early in the life of the tree and continued as needed. To minimize damage and promote rapid healing, branches should be pruned before they are 5 cm (2 in) d.b.h. A neat, clean cut should be made, being careful not to be cut into the branch collar (44). Ring shakes and dark bands of discolored wood were associated with 14 of 17 stubs that were "flush cut" (branch collar removed) 13 years earlier. Pruning young trees eliminates these problems, but if older trees are pruned, care must be taken not to remove the branch collars that form around the bases of dying and dead branches.
When trees are pruned during the dormant season (early spring just before the leaves appear is best), wounds tend to heal more rapidly and completely and sprouts from dormant buds near the wound are less likely to develop. If sprouts do develop, they should be removed promptly. No more than 25 percent of the live crown should be released in a single year, and at least 50 percent of the total tree height should be maintained in live crown (10).
Damaging Agents- Black walnut is damaged by a number of insects. In southern Illinois more than 300 insect species were found on black walnut (49). Even though many insects feed on black walnut, only a few are considered serious pests. Two of the most common defoliating insects are the walnut caterpillar (Datana integerrima) and the fall webworm (Hyphantria cunea). They are commonly found eating the leaves beginning in midsummer and continuing until September. Important boring insects are the ambrosia beetle (Xylosandrus germanus), which may introduce a Fusarium fungus into the tree, causing dieback and resprouting from the base of the tree; the flatheaded apple tree borer (Chrysobothris femorata), which feeds in the phloem and outer sapwood area as larvae and on the foliage as adults; the walnut curculio (Conotrachelus retentus), which damages developing nuts when the larvae bore into them and cause great losses during the so-called "June drop" of walnuts; and the walnut shoot moth (Acrobasis demotella), which damages the terminal buds in early spring when the larvae bore into the still unexpanded bud, causing multiple forks and crooks in the main stem. The pecan leaf casebearer (Acrobasis juglandis) is closely related to the walnut shoot moth but is a much less damaging pest of black walnut. Important sucking insects are aphids or plant lice (Monellia spp. and Monelliopsis spp.), which suck the juices from leaves and often deposit a sticky substance called "honey-dew" on the leaf surface that may turn black and prevent photosynthesis; and the walnut lace bug (Corythucha juglandis), which causes damage when the adults and nymphs suck the sap from the lower surfaces of walnut leaflets.
Black walnut is susceptible to only a few serious diseases, but their impact is significant. Two serious root rot diseases found in seedling nurseries are caused by the fungi Phytophthora citricola and Cylindrocladium spp. An important mold of stored seed and seedlings is associated with Penicillia and other normally saprophytic fungi (24). Walnut anthracnose, caused by the fungus Gnomonia leptostyla, is a leaf spot disease that begins during wet spring weather, although symptoms may not become visible until June or July (49). Another important foliage disease is target leafspot which is caused by the fungus Cristulariella pryamidalis and is responsible for premature defoliation (38). A newly discovered, serious leaf spot disease is caused by the fungus Mycosphaerella juglandis (24).
Important stem diseases caused by fungi are the Fusarium cankers caused by several species of Fusarium and the perennial target canker (Nectria galligena) commonly known as Nectria canker (49). Cankers usually occur on the main stem where a branch broke off and left an open wound.
Animals damage black walnut in several ways. Deer browse on buds and rub antlers against young trees. Mice and rabbits gnaw on the stems of young trees during the winter, and squirrels dig up and eat direct-seeded nuts and feed on green and mature nuts still on the trees. Perching birds break the terminal or new branches from the tree, and the yellow-bellied sapsucker drills holes through the bark during late winter or early spring (49). Some trees may be nearly girdled with peck holes.
Decay, dieback, and frost also cause damage. At times dieback and frost damage may be extensive. Late spring frosts kill succulent new growth and thus reduce height growth and destroy desirable form. Late winter warming periods sometimes cause walnut trees to break dormancy prematurely, resulting in freezing injury to the stem tissue (13,37).
Special Uses
The best known use of black walnut is for its lumber and veneer. The wood is used for fine furniture of all kinds, interior paneling, specialty products, and gunstocks.
The nuts of black walnut serve many purposes. The kernels provide food for wildlife and humans (45,52). Ground shells provide special products (12). During World War II, airplane pistons were cleaned with a "nut shell" blaster and this idea was carried into the auto industry; manufacturers used shells to deburr precision gears. Ground shell products are also used to clean jet engines, as additives to drilling mud for oil drilling operations, as filler in dynamite, as a nonslip agent in automobile tires, as an air-pressured propellant to strip paints, as a filter agent for scrubbers in smokestacks, and as a flourlike carrying agent in various insecticides.
Genetics
Population Differences
Black walnut contains great genetic variation for growth and survival, and an important part of this variation is related to geographic origin (8). Preliminary seed collection zones have been recommended (15). Geographic variation among stands is three to five times greater than local (within stands) variation for characteristics such as growth rate, dates of foliation and leaf drop, twig maturation, and degree of winter dieback (17). Genetic gains can be made through selection within a designated seed collection zone. Generally, trees from seed collected south of the planting site grow as fast or faster in height and diameter than trees from local or northern sources (7,9). Both duration and rate of growth are responsible for the growth differences. In 1969, trees from Mississippi and Texas seed sources planted in a southern Illinois plantation grew in height for 134 days compared to 93 days for trees from northern Illinois and Iowa sources (5). On the average, height growth continued 1 day longer for every 24 miles south of the planting site that seed was collected (6). Duration of diameter growth was less closely related. However, trees of southern origin grew fastest.
Flowering phenology, seed weight, kernel percent, nut crackability, foliage characteristics, grafting and budding compatibility, rooting capacity of layered trees in stool beds, autumn leaf retention, cold resistance, and growth rates vary widely among black walnut families (17).
More than 400 black walnut cultivars have been named and released during the past century. Twenty of the most popular, including origin and nut evaluations, are listed by Funk (18). Three timber-type walnut clones chosen for outstanding straightness, anthracnose resistance, or late spring foliation have been patented by Purdue University.
Hybrids
Wright (54) has pointed out that species that can cross within a genus usually have distinct (often adjacent) ranges, while species that occupy the same sites in the same regions develop barriers to hybridization. Juglans seems to follow this pattern; J. nigra and J. cinerea often grow together but apparently never cross naturally, while all other walnut species (at least in the western hemisphere) are almost completely isolated. Thus, easy crossing might be expected among the morphologically similar North America Rhysocaryon walnuts. One example is the "Royal" hybrid between J. nigra and J. hindsii produced by Burbank in about 1888. This hybrid begins to bear viable seed by age 5 and produces exceptionally large nuts (50). The hybrids are vigorous and have been recommended for timber areas. Black walnut has been crossed with other species of Juglans in attempts to increase nut production, to produce a thin-shelled nut, or to produce a faster growing tree. Juglans can be divided into three sections: the black walnuts, the butternuts, and the Persian/Carpathians. A somatic chromosome number of 32 is consistent for all the species reported to date (18).
Crossing between the black walnut and butternut sections is difficult or impossible. A cross between J. nigra and J. ailantifolia is the only one recognized between the black walnut and butternut sections. However, J. regia can hybridize with species in both the other sections, although the crosses are not always easy.
Artificial hybridization is simple but time consuming. Each pollination may yield two or three nuts and a season's work only a few thousand nuts.
Literature Cited
- Ashley, Burl. 1977. Revisiting a 14-year old plantation. USDA Forest Service, Black Walnut Advisory Sheet 41. Northeastern Area State and Private Forestry, Morgantown, WV. 2 p.
- Baker, Frederick S. 1948. A revised tolerance table. Journal of Forestry 47:179-181.
- Beineke, Walter F. 1974. Recent changes in the population structure of black walnut p. 43-46. In Proceedings, Eighth Central States Forest Tree Improvement Conference, University of Missouri, Columbia, MO.
- Beineke, Walter F., and Michael N. Todhunter. 1980. Grafting black walnut. Purdue University, Forestry Note R105. West Lafayette, IN. 3 p.
- Bey, Calvin F. 1971. Early growth of black walnut trees from twenty seed sources. USDA Forest Service, Research Note NC105. North Central Forest Experiment Station, St. Paul, MN. 4 p.
- Bey, Calvin F. 1973. Genetic variation and selection. In Black walnut as a crop. Proceedings, Black Walnut Symposium, August 14-15, 1973, Carbondale, IL. p. 62-65. USDA Forest Service, General Technical Report NC-4. North Central Forest Experiment Station, St. Paul, MN.
- Bey, Calvin F. 1973. Growth of black walnut trees in eight midwestern States-a provenance test. USDA Forest Service, Research Paper NC-91. North Central Forest Experiment Station, St. Paul, MN. 7 p.
- Bey, Calvin F. 1980. Growth gains from moving black walnut provenances northward. Journal of Forestry 78(10):640-645.
- Bey, Calvin F., and R. D. Williams. 1975. Black walnut trees of southern origin growing well in Indiana. Indiana Academy of Science Proceedings 84(1974):122-127.
- Brinkman, Kenneth A. 1965. Black walnut (Juglans nigra L.). In Silvics of forest trees of the United States. H. A. Fowells, comp. U.S. Department of Agriculture, Agriculture Handbook 271. 762 p.
- Burke, Robert D., and R. D. Williams. 1973. Establishment and early culture of plantations. In Black walnut as a crop. Proceedings, Black Walnut Symposium, August 14-15, 1973, Carbondale, IL. p. 36-41. USDA Forest Service, General Technical Report NC-4. North Central Forest Experiment Station, St. Paul, MN.
- Cavender, Clarence C. 1973. Utilization and marketing of shells. In Black walnut as a crop. Proceedings, Black Walnut Symposium, August 14-15, 1973, Carbondale, IL. p. 77-78. USDA Forest Service, General Technical Report NC-4. North Central Forest Experiment Station, St. Paul, MN.
- Clark, F. Bryan. 1961. Climatic injury found on planted black walnut in Kansas. USDA Forest Service, Station Note 147. Central States Forest Experiment Station, St. Paul, MN. 2 p.
- Clark, Paul M., and Robert D. Williams. 1979. Black walnut growth increased when interplanted with nitrogen-fixing shrubs and trees. Indiana Academy of Science Proceedings 88:88-91.
- Deneke, Frederick J., David T. Funk, and Calvin Bey. 1980. Preliminary seed collection zones for black walnut. USDA Forest Service, NA-FB/M-4. Northeastern Area State and Private Forestry, Broomall, PA. 5 p.
- Eyre, F. H., ed. 1980. Forest cover types of the United States and Canada. Society of American Foresters, Washington, DC. 148 p.
- Funk, David T. 1970. Genetics of black walnut. USDA Forest Service, Research Paper WO-10, Washington, DC. 13 p.
- Funk, David T. 1979. Black walnuts for nuts and timber. In Nut tree culture in North America. p. 51-73. Northern Nut Growers Association, Hamden, CT.
- Funk, David T., P. Case, W. J. Rietveld, and Robert E. Phares. 1979. Effects of juglone on the growth of coniferous seedlings. Forest Science 25(3):452-454.
- Geyer, Wayne A., Robert D. Marquard, and Joel F. Barber. 1980. Black walnut site quality in relation to soil and topographic characteristics in northeastern Kansas. Journal of Soil and Water Conservation 35(3):135-137.
- Grey, Gene W., and Gary G. Naughton. 1971. Ecological observations on the abundance of black walnut in Kansas. Journal of Forestry 69(10):741-743.
- Kaeiser, Margaret, Jay H. Jones, and David T. Funk. 1965. Interspecific walnut grafting in the greenhouse. The Plant Propagator 2021(4/1):2-7.
- Kellogg, L. F. 1940. Yield of plantation black walnut in the Central States Region. USDA Forest Service. Unpublished report. 166 p.
- Kessler, Kenneth J. 1981. Personal communication. Carbondale, IL.
- Krajicek, John E. 1975. Planted black walnut does well on cleared forest sites-if competition is controlled. USDA Forest Service, Research Note NC-192. North Central Forest Experiment Station, St. Paul, MN. 4 p.
- Krajicek, John E., and Robert D. Williams. 1971. Continuing weed control benefits young planted black walnut. USDA Forest Service, Research Note NC-122. North Central Forest Experiment Station, St. Paul, MN. 3 p.
- Lamb, George N. 1915. A calendar of the leafing, flowering and seeding of the common trees of the eastern United States. Monthly Weather Review, Suppl. 2, Pt 1. 19 p.
- Landt, Eugene F., and Robert E. Phares. 1973. Black walnut ... an American wood. USDA Forest Service, FS-270. Washington, DC. 7 p.
- Little, Elbert L., Jr. 1971. Atlas of United States trees, vol. 1. Conifers and important hardwoods. U.S. Department of Agriculture, Miscellaneous Publication 1146. Washington, DC. 9 p., 313 maps.
- Losche, Craig K. 1973. Black walnut growth better on deep, well-drained bottomland soils. USDA Forest Service, Research Note NC-154. North Central Forest Experiment Station, St. Paul, MN. 3 p.
- Losche, Craig K. 1973. Selecting the best available soils. In Black walnut as a crop. Proceedings, Black Walnut Symposium, August 14-15, 1973, Carbondale, IL. p. 33-35. USDA Forest Service, General Technical Report NC-4. North Central Forest Experiment Station, St. Paul, MN.
- MacDaniels, L. H., and David L. Pinnow. 1976. Walnut toxicity, an unsolved problem. Northern Nut Growers Association Annual Report 67(1976):114-122.
- McKay, J. W. 1956. Walnut blossoming studies in 1956 Northern Nut Growers Association Annual Report 47:79-82.
- Masters, Charles J. 1974. The controlled pollination techniques and analysis of intraspecific hybrids for black walnut. Thesis (Ph.D.), Purdue University, Department of Forestry and Natural Resources, West Lafayette, IN. 122 p.
- Masters, Charles J., and Walter F. Beineke. 1973. Clonal vs. half-sib orchards for black walnut. In Proceedings, Twentieth Northeastern Forest Tree Improvement Conference, July 1972, Durham, NH. p. 52-61. USDA Forest Service, Northeastern Forest Experiment Station, Broomall, PA.
- Melichar, M. W., H. E. Garrett, and G. S. Cox. 1982. A screening of vesicular-arbuscular (VA) mycorrhizal forming fungi on black walnut seedlings. In Black walnut for the future. p. 118-121. USDA Forest Service, General Technical Report NC-74. North Central Forest Experiment Station, St. Paul, MN. 152 p.
- Murray, Gordon, and William R. Brynes. 1975. Effect of night temperature on dehardening in black walnut seedlings. Forest Science 21(3):313-317.
- Neely, Dan, Robert Phares, and Barbara Weber. 1976. Cristulariella. leaf spot associated with defoliation of black walnut plantations in Illinois. Plant Disease Reporter 60(7):588-590.
- Phares, Robert E., and Robert D. Williams. 1971. Crown release promotes faster diameter growth of pole-size black walnut. USDA Forest Service, Research Note NC-124. North Central Forest Experiment Station, St. Paul, MN 4 p.
- Ponder, Felix, Jr. 1979. Fertilization and release increase nut production of pole size black walnut. p. 138-143. In Flowering and seed development in trees: Proceedings of a Symposium, May 1978, Starkville, Mississippi. Frank Bonner, ed. Southern Forest Experiment Station, Starkville, MS. 380 p.
- Ponder, Felix, Jr., R. C. Schlesinger, and D. T. Funk. 1980. Autumn olive stimulates growth of black walnut. Southern Lumberman 240:14-16.
- Rietveld, W. J. 1979. Ecological implications of allelopathy in forestry. In Regenerating oaks in upland hardwood forests, Proceedings, 1979 J. S. Wright Forestry Conference. p. 91-111. Purdue University, West Layfette, IN.
- Schlesinger, Richard C., and David T. Funk. 1977. Manager's handbook for black walnut. USDA Forest Service, General Technical Report NC-38. North Central Forest Experiment Station, St. Paul, MN. 22 p.
- Shigo, Alex L., E. A. McGinnes, Jr., D. T. Funk, and N. Rogers. 1979. Internal defects associated with pruned and nonpruned branch stubs in black walnut. USDA Forest Service, Research Paper NE-440. Northeastern Forest Experiment Station, Broomall, PA. 27 p.
- Smith, Christopher C., and David Follmer. 1972. Food preferences of squirrels. Ecology 53(l):82-91.
- U.S. Department of Agriculture, Forest Service. 1974. Seeds of woody plants in the United States. p. 454-459. C. S. Schopmeyer, tech. coord. U.S. Department of Agriculture, Agriculture Handbook 450. Washington, DC.
- U.S. Department of Agriculture, Forest Service. 1980. Root characteristics of some important trees of eastern forests: a summary of the literature. USDA Forest Service, Eastern Region, Milwaukee, WI. 217 p.
- von Althen, F. W. 1971. Extended stratification assures prompt walnut germination. The Forestry Chronicle, December 1971, p. 349.
- Weber, Barbara C., Robert L. Anderson, and William H. Hoffard. 1980. How to diagnose black walnut damage. USDA Forest Service, General Technical Report NC-57. North Central Forest Experiment Station, St. Paul, MN. 20 p.
- Whitson, John, Robert John, and Henry Smith Williams, eds. 1915. The production of a quick-growing walnut. In Luther Burbank-his methods and discoveries and their practical application. Chap. 11, p. 193-237. Luther Burbank Press, New York and London.
- Williams, Robert D. 1972. Root fibrosity proves insignificant in survival, growth of black walnut seedlings. Tree Planters' Notes 23(3):22-25.
- Williams, Robert D. 1979. Cow manure deters rodents from stealing seeded black walnut. Northern Nut Growers Association Annual Report 69:43-48.
- Williams, Robert D. Data from weed control study on file at North Central Forest Experiment Station, Bedford, IN.
- Wright, Jonathan W. 1962. Genetics of forest tree improvement. FAO Forest and Forest Products Series 16. Rome, Italy. 399 p.
Source: https://www.srs.fs.usda.gov/pubs/misc/ag_654/volume_2/juglans/nigra.htm
0 Response to "How to Draw a Black Wallnut Tree Leaf"
Post a Comment